Silanes
Handling Precautions
Quality, Storage and Handling
- The products should be kept in a cool, dark, and dry place.
- The coupling agents may deteriorate when in contact with water or moisture, producing byproducts such as hydrogen chloride from chlorosilanes and methanol or ethanol from methoxy silanes or ethoxysilanes, respectively. These products should be handled with special care when kept in the open air. After opening, they should be tightly sealed to limit exposure to water or moisture. It is recommended that dry nitrogen be used to replace the air in opened containers.
- Please contact our Sales Department for the SDS and read it carefully before using any of these products.
Safety
- These products should be handled with adequate ventilation to avoid contact with water or moisture. For example, when KA-1003 reacts with water or moisture in the air, it may generate corrosive hydrogen choloride gas, which is harmful to skin and membranes.
- To avoid contact with skin or membranes, it is recommended to wear gloves and goggles for protection. If contact occurs, flush immediately with large amounts of water.
- When eye contact occurs, flush the eyes immediately with large amounts of water and consult a doctor, if necessary. In the case of KA-1003 or aminosilanes, special and prompt care is required.
- If contact with clothes occurs, flush the exposed clothing with water and then wash the clothes immediately.
- After using silanes, wash hands very thoroughly before eating or drinking.
- If silane fluids are spilled, either flush the exposed area with large amounts of water or clean with rags or sand, which should be promptly disposed of by burning.
FAQ
- We understand that the solutions of silane coupling agent ares diluted when silane coupling agents are applied. Howare the solutions made?
Silanes are usually diluted with water to a concentration of approximately 0.1 - 2.0 %. If using silanes that are not soluble in water, a combination of 0.1 - 2.0 % of acetic acid in water or water-alcohol (acetic acid, water, and alcohol together) is recommended. Acetic acid is used to control hydrolysis rates. Adjustment of the pH greatly influences the stability of silanols.
How to prepare standard diluted solutions
- Prepare the water solution by adding acetic acid to a final concentration of 0.1 - 2.0 %. If the silanes are more soluble, a lower concentration of acetic acid is recommended. For aminosilanes, there is no need to add acetic acid.
- Mix the acetic acid solution while dropping the silanes to achieve a final silane concentration of approximately 0.1 - 2.0 %. By mixing the solution rapidly while dropping the silanes slowly, gel formation can be avoided.
- After adding the silane, stirring should be continued for another 30~60 minutes until the solution becomes transparent, indicating that the hydrolysis of the silane is complete.
- Filtration should follow, if necessary. Filtration is recommended if solid impurities occur. If the silane solution is to be used continuously, a cycle of filtration with a cartridge below 0.5 µm is recommended.
- The stability of each Shine-Etsu silane coupling agent is given below.
The alkoxysilyl group reacts with water to yield a silanol group that is not stable and will condense rapidly to form a siloxane structure. A silanol is usually not stable in the presence of water, but it is more stable in weakly acidic solutions. Aminosilanes are an exception since the amino group helps the silane to become more stable in water solutions. The following table gives information concerning water solutions of several products and their most stable pH values.
Product name Solubility
(pH of aqueous solution)Shelf-life KBM-1003 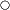 (3.9)
(3.9)Up to 10 days KBE-1003 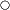 (3.9)
(3.9)Up to 10 days KBM-303 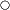 (4.0)
(4.0)Up to 30 days KBM-403 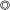 (5.3)
(5.3)Up to 30 days KBE-402 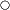 (4.0)
(4.0)Up to 10 days KBE-403 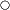 (4.0)
(4.0)Up to 10 days KBM-1403 Insoluble - KBM-502 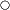 (4.0)
(4.0)Up to 1 days KBM-503 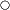 (4.2)
(4.2)Up to 1 days KBM-5103  (4.2)
(4.2)Up to 3 days KBM-602  (10.0)
(10.0)Up to 30 days KBM-603  (10.0)
(10.0)Up to 30 days KBM-903  (10.0)
(10.0)Up to 30 days KBE-903 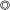 (10,0)
(10,0)Up to 30 days KBM-573 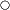 (4.0)
(4.0)Up to 1 days KBM-803 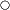 (4.0)
(4.0)Up to 1 days  : 1% silane-water solution can be prepared without adjusting pH of aqueous solution
: 1% silane-water solution can be prepared without adjusting pH of aqueous solution
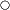 : 1% silane-water solution can be prepared if pH of aqueous solution is adjusted
: 1% silane-water solution can be prepared if pH of aqueous solution is adjusted
Insoluble: silane-water solution cannot be prepared
Basic Structure
Silane coupling agents have two different types of reactive functional groups.
Functions
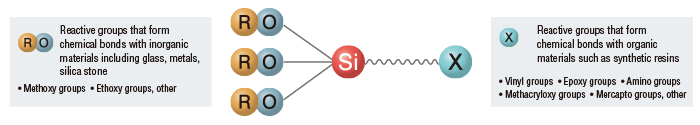
Inorganic Reactive Silanes
In the presence of water, coupling agents produce highly reactive silanols. Subsequently, these silanols begin to condense, forming oligomeric structures while also forming weak hydrogen bonds to the surface of inorganic materials. Finally, drying the inorganic materials leads to further condensation and dehydration between the coupling agent and the surface. This process yields multiple strong, stable, covalent bonds to the surface.
Organic Reactive Silanes
The improved adhesion between the surfaces of inorganic materials treated with silane coupling agents and organic resins is caused by:
- Improved wetting of the treated inorganic surface by the resin.
- Improved compatibility between the treated inorganic surface and the resin.
- Hydrogen bonding between the treated inorganic surface and the resin.
- Multiple covalent bonds between the treated inorganic surface and the resin.
Many different factors affect the above four items, such as the type of thermoplastic or thermosetting resins, whether or not functional groups remain, the abundance and reactivity of the remaining functional groups, and the overall polarity or non-polarity of the resins.
- Thermoplastic resins
For thermoplastics, the chemical bonds introduced by reactive silanes are often relatively weak. A limited number of highly polar thermoplastics will develop weak interactions with some silane coupling agents. In these specific situations, both the thermoplastic resin and the silane are capable of forming hydrogen bonds. Therefore, the effectiveness of resin modification is highly dependent on the compatibility of each organic resin and its ability to form hydrogen bonds. - Thermosetting resins
Unlike thermoplastics where considerations such as the critical surface tension, the dissolution parameters, and other similar factors may be used to evaluate resin compatibility, these factors are not meaningful for predicting the strength of composite materials prepared from thermosetting resins. To maximize the strength and other physical properties of a thermosetting composite, it is generally recommended to first react the organic functional group of the silane with the thermosetting resin before curing the composite. It is important to use a reactive silane coupling agent bearing an appropriate functional group that matches the functional reactivity of the thermosetting resins.
How to Use
There are two basic approaches for using silane coupling agents. The silane can either be used to treat the surface of the inorganic materials before mixing with the organic resin or it can be added directly to the organic resin.
The Surface Treatment of Inorganic Materials
There are two general methods for treating the surfaces of inorganic filler materials before they are added to the organic resins.
(1) Wet Method
By mixing a slurry of the inorganic materials in a dilute solution of the silane coupling agent, a highly uniform and precise surface treatment of the inorganic material can be obtained.(2) Dry Method
A high shear, high speed, mixer is used to disperse the silane coupling agent into the inorganic materials. The silane is generally applied either neat or as a concentrated solution. When compared to the Wet Method, the Dry Method is most often preferred for large-scale production, treating a large amount of filler in a relatively short time and generating relatively little mixed waste; however, it is more difficult to obtain uniform treatment with this method.Addition To Organic Materials
Compared to the methods for the surface treatment of inorganic materials, adding the silane to the organic resin is more widely used in industries because of its excellent process efficiency, although curing may be more difficult. There are two general methods.
(1) Integral Blending
This method involves simple blending of the silane coupling agent into the composite formula as the inorganic and organic materials are mixed together.(2) Master Batch
In this method, the silane coupling agent is first added to a small amount of the organic resin material to form what is referred to as a "master batch". Usually in the form of pellets or large granules, the master batch can be easily added along with the pellets of the organic resin when producing the composite materials.
Applications
Thermosetting Resins
(1) Glass fiber reinforced epoxy resins
In order to meet the electrical properties and heat resistance requirements of epoxy resin laminated plates used with molten solder alloys, silane coupling agents are recommended as a resin modifier for the thermosetting composites. In this case, silane coupling agents are generally used to treat glass fibers that have been pre-treated with a water solution and then dipped in a resin vanish.(2) Encapsulating semiconductors
The most common use for coupling agents in epoxy molding compounds is as a semiconductor sealing agent that improves the moisture resistance and electrical characteristics of the resultant composite materials. The coupling agents form an interfacial bond between the resins and the filler that is stronger and more hydrolytically stable, yielding a better moisture resistant interface. In this case, volume resistivity and bending strength are also greatly improved.(3) Coated sand
The casting parts are comprised of fire resistant aggregates (sand) and adhesives. The quality of the resultant casting is reflects the strength of the adhesives coated on the surface of the sand particles. The coupling agents play an important role by improving the strength of the cast as well as preventing moisture. In most cases, the coupling agents are pre-added directly to the resins.Thermoplastics
The results obtained from using coupling agents in thermoplastic resins are generally lower than when compared with that of thermosetting resins. However, in a limited number of systems such as nylon and plastic magnets, good results are achieved due to the high polarity of the thermoplastic resins that are used.Resin modification
The uses of silane coupling agents are not limited to the interfaces of composite materials. Resin modification can create high performance resins with unique and superior characteristics. Typically, resins modified with silanes display improved adhesion to inorganic materials and moisture curable properties at low temperature, as well as superior resistance to weathering, acid, heat, and solvents. Product development continues, including the applications of polyolefins for electrical wires and acrylic resins for modified sealants.For resin modifications with the silicon-based compounds, the following reactions are possible:
(1) Grafting

Grafting is widely used to produce polyolefin based materials for sealing electrical wires. Polyolefins that incorporate an unsaturated silane couplant (e.g., vinyltrimethoxysilane, γ-methacryloxypropyltrimethoxysilane, etc.) have a silyltrimethoxy group grafted to the polyolefin backbone that enables moisture crosslinkable resins. Moisture crosslinkable polyolefins are highly preferred for electrical wire applications because of their reasonable cost and excellent electrical insulation, as well as their dielectric and mechanical stability. In these applications, common silanol condensation catalysts such as dibutyltindilaurate, dibutyltindioleate, dibutyltindiacetate, tetrabutyltitanate, and stannous octanoic acid are used in conjunction with the a peroxide for the grafting reaction.
(2) Chemical Reactions

Given the variety of silane coupling agents that are available bearing different organic functional groups as well as the many different types of organic resins produced, a large number of chemical reactions can be developed between using these compounds as reactants. Examples of applications for this type of silane modified resins include modified sealants, where polyoxyalkylene resins bearing a terminal aryl group react with a hydrosilane in the presence of platinum catalyst, and moisture curable urethane resins, where thermoplastic urethane resins have been modified by an amino functional alkoxysilane. These types of methods for resin modification are expected to continue to produce new resins in the future.
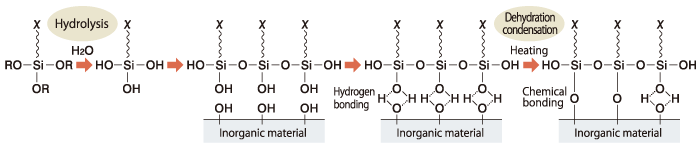
(3) Copolymerization
Copolymerization of an unsaturated silane monomer along with one or more organic monomers is widely used to modify acrylic resins for paints. This method often uses a silane couplant with a methacrylic functional group with compatible co-monomers.
Key Products

KBE-9007N
KBE-9007N is recommended to improve the adhesion of composite materials.

KBM-5103
KBM-5103, a new product with a reactive acryloxy functional group, is designed to significantly improve the adhesion and reinforcement of materials.

KBE-9103P
KBE-9103P is used as an additive to epoxy or phenolic systems that would not otherwise allow a one-component system due to reactivity or incompatibility with aminosilanes.
