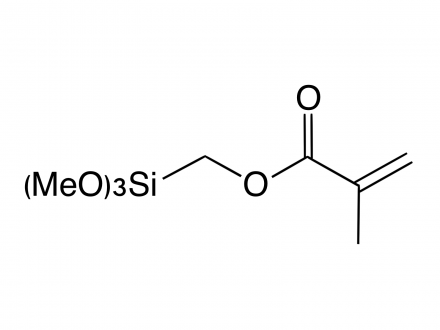
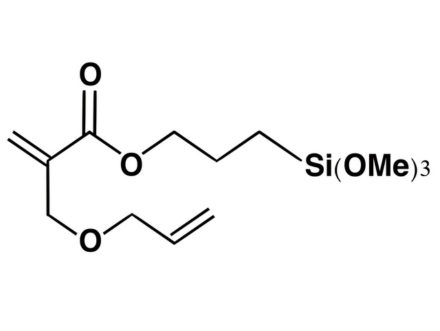
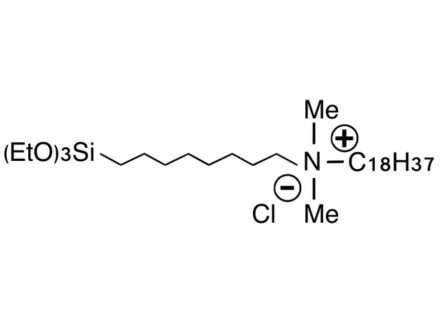
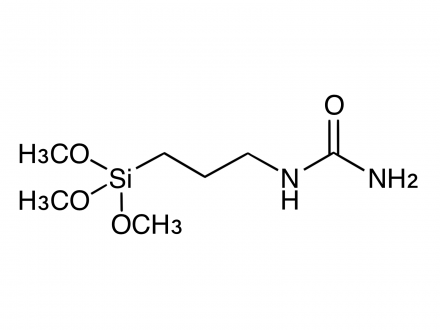
What is the difference in use of methoxysilyl and ethoxysilyl groups in silane coupling agents?
The hydrolysis of alkoxysilyl groups is faster for methoxysilyl groups than for ethoxysilyl groups.
Hydrolytic Activity of Alkoxy Groups
In general, the methoxy group (-OCH₃) is more reactive (hydrolyzable) than the ethoxy group (-OC₂H₅).
Under acidic conditions, the reaction is faster with fewer alkoxy groups, so the order of hydrolysis speed is as follows;
Dimethoxy > Trimethoxy > Diethoxy > Triethoxy.
On the other hand, under basic conditions, the order is as follows;
Trimethoxy > Dimethoxy > Triethoxy > Diethoxy.
![]()
Hydrolysis of the methoxysilyl group produces methanol, and hydrolysis of the ethoxysilyl group produces ethanol, so if you are concerned about the generation of methanol, please use the ethoxysilyl group (KBE series).
Related FAQ
- How to store pretreated inorganic materials or silane-doped resins?
- How to increase the solubility of silane coupling agent in water and improve the storage stability of the solution?
- How to store silane coupling agents pretreated on inorganic materials or resins with silane added?
- How to store the solution after hydrolyzing the silane coupling agent?
- What precautions should be taken when storing silane coupling agents?